MEDICAL VACUUM PLANT
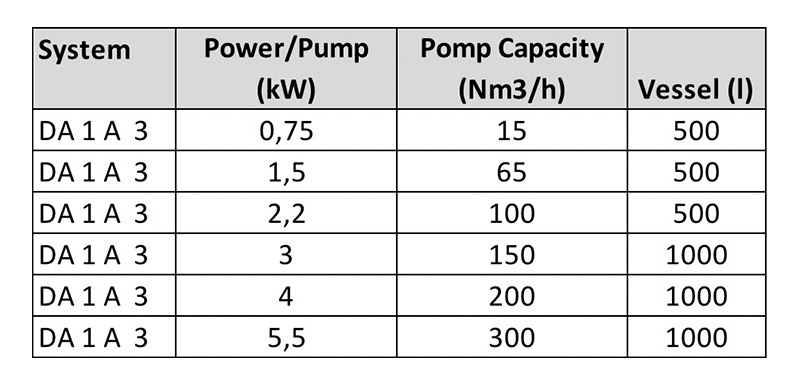
Technical specifications
MEDICAL VACUUM SYSTEM by Master Energia
Medical vacuum employs almost the same methods and equipment used in industrial vacuum systems. Yet, it is produced according to the harmonised EN ISO Standard 7396-1 and to the way the norm applies to central vacuum systems for surgical endocavitary suction.
The main difference lies in the high levels of hygiene the system needs to guarantee. It features special antibacterial filters, which trap the widest range of impurities. Another prerequisite of medical vacuum systems is the use of different auxiliary emergency systems: secondary pumps and filters are activated automatically in case of failure in the primary elements. Stainless steel AISI 316L is the ideal material to guarantee high standards of hygiene: unlike other materials, it is neutral and bacteria do not proliferate over steel surfaces. Central Medical Vacuum Supply System is used to provide a reliable and continuous suction in various departments of a hospital, such as operation theater, intensive care- unit, coronary and neonatal care-unit, delivery and emergency departments and patient wards.
A scavenging system includes a vacuum generator to start the system, a pipeline system and one or multiple terminal units. Specific relief valves for waste gas are connected to the anaesthetic gas suction system.
An active scavenging system is to be preferred over a passive one. In compliance with UNI EN Standard 7396, the medical vacuum system consists of a primary and a secondary vacuum pump, an emergency vacuum pump and a tank to make sure the flow is always constant and pulse-free.
The medical vacuum systems manufactured by Master Energia complies with Directive 93/42/CEE and pre-sents all technical requirements established by national and international standards. Regardless of the manufacturing site where they are produced, all components undergo thorough checks. Then, they are assembled in-house and tested. All medical devices manufactured by the company are CE marked, in compliance with the quality requirements indicated in EN ISO Standard 13485.


